A. Atomic Absorption Spectrophotometer
 An atomic absorption spectrophotometer analyzes the concentration of elements in a liquid sample based on energy absorbed from certain wavelengths of light (usually 190 to 900 nm). Atomic absorption spectrophotometers typically include a flame burner to atomize the sample (most commonly a hollow cathode lamp), a monochromator, and a photon detector. Depending on the model, some atomic absorption spectrometers are equipped with a turret or fixed lamp socket that can hold multiple lamps (up to eight) to reduce downtime between samples or allow for sequential analysis.
An atomic absorption spectrophotometer analyzes the concentration of elements in a liquid sample based on energy absorbed from certain wavelengths of light (usually 190 to 900 nm). Atomic absorption spectrophotometers typically include a flame burner to atomize the sample (most commonly a hollow cathode lamp), a monochromator, and a photon detector. Depending on the model, some atomic absorption spectrometers are equipped with a turret or fixed lamp socket that can hold multiple lamps (up to eight) to reduce downtime between samples or allow for sequential analysis.
Typical sensitivity for an atomic absorption spectrometer using a flame burner is in the parts per million range. For trace analysis, a graphite furnace can be used in place of a flame burner to increase the sensitivity by several orders of magnitude (in the parts per billion range). Atomic absorption spectrophotometers are used in many industries including environmental testing, metal analysis, semiconductor manufacturing, petroleum and chemical production, and in pharmaceuticals.
Manufacturer are invited to advertise their product photo free of cost
B. UV visible spectrometer
 Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared (NIR)) ranges. The absorption in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.
Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared (NIR)) ranges. The absorption in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.
Manufacturer are invited to advertise their product photo free of cost
C. Inductively coupled atomic emission spectrophotometer
 Inductively coupled plasma atomic emission spectroscopy (ICP-AES), also referred to as inductively coupled plasma optical emission spectrometry (ICP-OES), is an analytical technique used for the detection of trace metals. It is a type of emission spectroscopy that uses the inductively coupled plasma to produce excited atoms and ions that emit electromagnetic radiation at wavelengths characteristic of a particular element.[1][2] The intensity of this emission is indicative of the concentration of the element within the sample.
Inductively coupled plasma atomic emission spectroscopy (ICP-AES), also referred to as inductively coupled plasma optical emission spectrometry (ICP-OES), is an analytical technique used for the detection of trace metals. It is a type of emission spectroscopy that uses the inductively coupled plasma to produce excited atoms and ions that emit electromagnetic radiation at wavelengths characteristic of a particular element.[1][2] The intensity of this emission is indicative of the concentration of the element within the sample.
Manufacturer are invited to advertise their product photo free of cost
D. X-ray fluorescence spectrophotometer
 X-ray fluorescence spectrometers (XRFs) use a spectroscopic technique that is commonly used with solids, in which X-rays are used to excite a sample and generate secondary X-rays. The X-rays broadcast into the sample by X-ray fluorescence spectrometers eject inner-shell electrons. Outer-shell electrons take the place of the ejected electrons and emit photons in the process. The wavelength of the photons depends on the energy difference between the outer-shell and inner-shell electron orbitals. The amount of X-ray fluorescence is very sample dependent and quantitative analysis requires calibration with standards that are similar to the sample matrix.
X-ray fluorescence spectrometers (XRFs) use a spectroscopic technique that is commonly used with solids, in which X-rays are used to excite a sample and generate secondary X-rays. The X-rays broadcast into the sample by X-ray fluorescence spectrometers eject inner-shell electrons. Outer-shell electrons take the place of the ejected electrons and emit photons in the process. The wavelength of the photons depends on the energy difference between the outer-shell and inner-shell electron orbitals. The amount of X-ray fluorescence is very sample dependent and quantitative analysis requires calibration with standards that are similar to the sample matrix.
The solid samples used with X-ray fluorescence spectrometers are usually powdered and pressed into a wafer or fused in a borate glass. The sample is then placed in the sample chamber of the XRF spectrometer, and irradiated with a primary X-ray beam. The X-ray fluorescence is measured either in simultaneous or sequential modes, and is recorded with either an X-ray detector after wavelength dispersion or with an energy-dispersive detector.
X-ray fluorescence spectrometers measure the emitted X-ray fluorescence in either a simultaneous fashion, or sequential. Simultaneous mode typically measures the entire wavelength range around the emission line of interest simultaneously, while sequential mode typically measures one wavelength at time.
Wavelength dispersive detectors use a nondestructive analysis technique for the identification and quantification of elements in a material. Wavelength dispersive spectroscopy is the measurement x-ray energies emitted from the bombardment of an energy source impinged upon the material, producing characteristic x-rays. Energy dispersive X-ray fluorescence spectrometers also use nondestructive analysis techniques for the identification and quantification of elements in a material. Energy dispersive spectroscopy is the measurement x-ray energies emitted from the bombardment of an energy source impinged upon the material, producing characteristic x-rays.
XRF was originally used to analyze geological samples. The advancement of computers and other technologies allowed the technique to develop even further. XRF found its place in many different types of analytical laboratories and some industrial inspection systems. X-ray fluorescence spectrometers provide a number of distinct advantages including easy sample preparation, nondestructive rapid multi-element analysis, and the ability to screen unknowns in a wide array of sample matrices such as liquids, solids, slurries, powders, pastes, thin films, air filters, and many others. Because of these advantages the technique, it is widely used for research, in industrial settings, and by quality assurance analysts.
Manufacturer are invited to advertise their product photo free of cost
E. XRD spectrophotometer
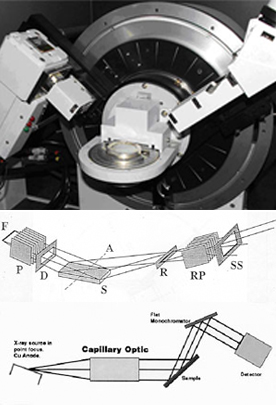 X-ray diffraction (XRD) is a versatile, non-destructive technique that reveals detailed information about the chemical composition and crystallographic structure of natural and manufactured materials.
X-ray diffraction (XRD) is a versatile, non-destructive technique that reveals detailed information about the chemical composition and crystallographic structure of natural and manufactured materials.
Crystal lattice
A crystal lattice is a regular three-dimensional distribution (cubic, rhombic, etc.) of atoms in space. These are arranged so that they form a series of parallel planes separated from one another by a distance d, which varies according to the nature of the material. For any crystal, planes exist in a number of different orientations - each with its own specific d-spacing.
Constructive interference
When a monochromatic X-ray beam with wavelength lambda is projected onto a crystalline material at an angle theta, diffraction occurs only when the distance traveled by the rays reflected from successive planes differs by a complete number n of wavelengths.
Bragg's Law
By varying the angle theta, the Bragg's Law conditions are satisfied by different d-spacings in polycrystalline materials. Plotting the angular positions and intensities of the resultant diffracted peaks of radiation produces a pattern, which is characteristic of the sample. Where a mixture of different phases is present, the resultant diffractogram is formed by addition of the individual patterns.
Based on the principle of X-ray diffraction, a wealth of structural, physical and chemical information about the material investigated can be obtained. A host of application techniques for various material classes is available, each revealing its own specific details of the sample studied.
Manufacturer are invited to advertise their product photo free of cost
F. Discharge Optical Emission Spectroscopy - GDOES
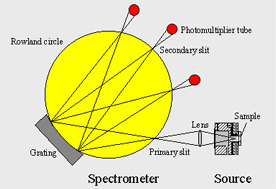 Glow Discharge Optical Emission Spectrometry (GDOES) is a well established method for the analysis of in-depth concentration profiles By this method, it is possible to perform fast simultaneous analysis of many elements, in a wide range of concentrations and depths. Especially in the field of galvanised applications GDOES shows a very good performance in respect of the speed of analysis as well as the quality of the chemical analysis.
Glow Discharge Optical Emission Spectrometry (GDOES) is a well established method for the analysis of in-depth concentration profiles By this method, it is possible to perform fast simultaneous analysis of many elements, in a wide range of concentrations and depths. Especially in the field of galvanised applications GDOES shows a very good performance in respect of the speed of analysis as well as the quality of the chemical analysis.
GDOES combines sputtering and atomic emission to provide an extremely rapid and sensitive technique for element depth profiling. During analysis, a plasma is generated in the analysis chamber by the applied voltage between the anode and the cathode (the sample surface) in the presence of argon under low pressure. Ionised Ar atoms cause sputtering of the sample area. Sputtered atoms excited in the plasma rapidly de-excite by emitting photons with characteristic wavelengths
Manufacturer are invited to advertise their product photo free of cost
Note :- Above Photos & Presentation are used for Representation Purpose only not for commercial aspect
